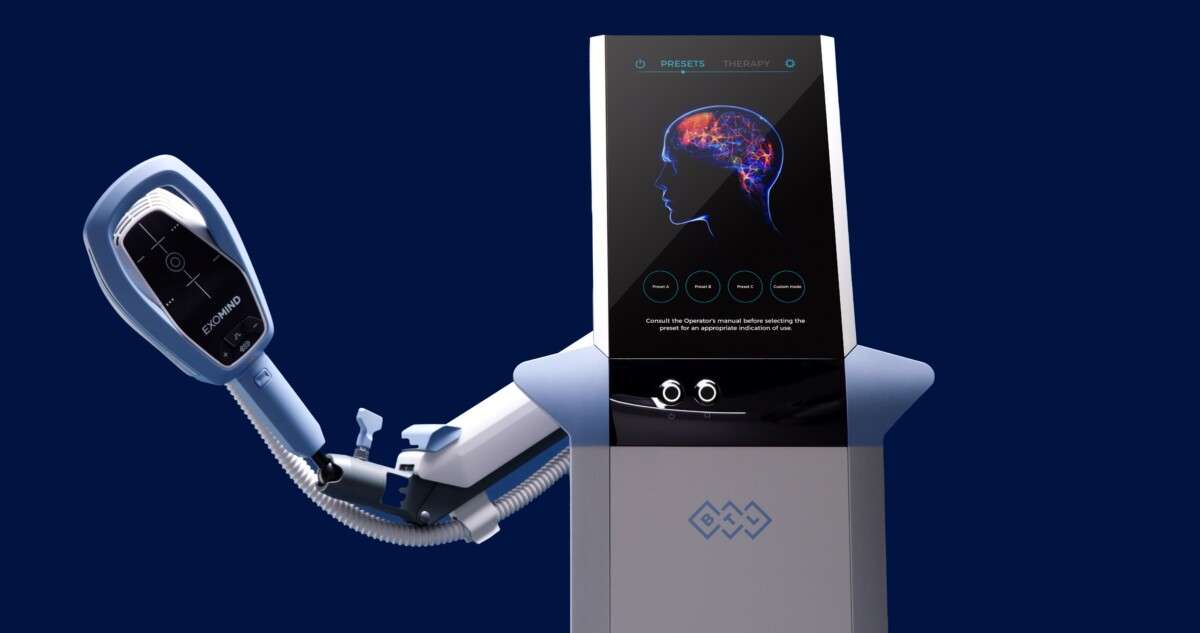

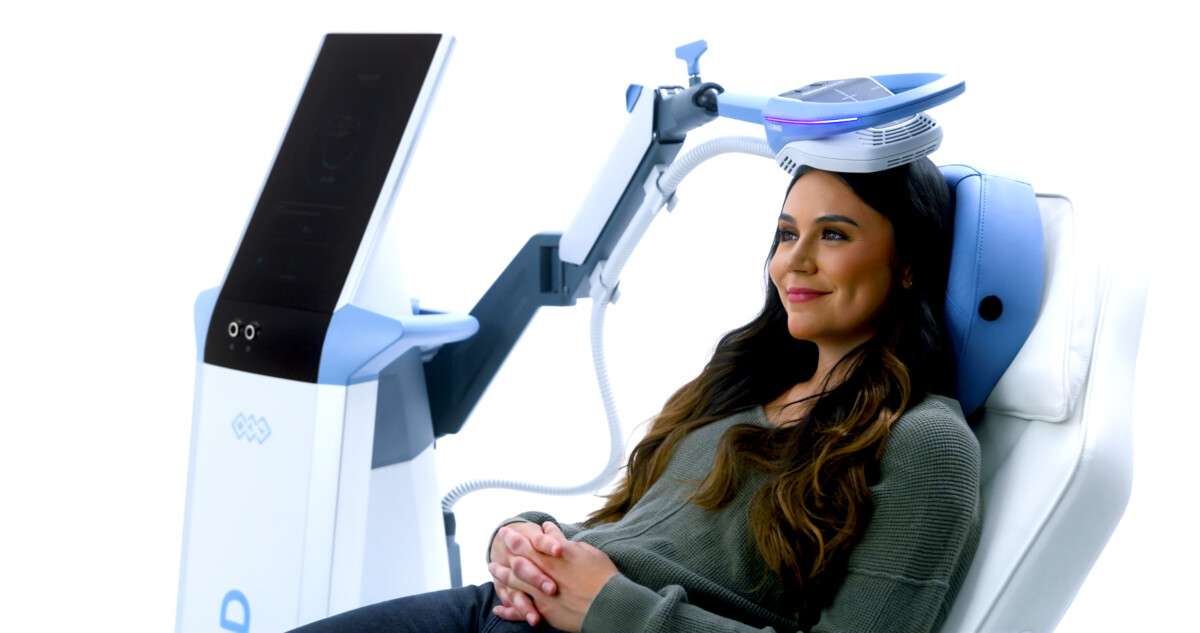
We proudly introduce EXOMIND™, BTL Industries’ newest innovation and a groundbreaking addition to our wellness offerings.
Using proprietary ExoTMS technology, this FDA-cleared treatment delivers precise, non-invasive magnetic stimulation to targeted areas of the brain, restoring healthy function, promoting emotional resilience, and reducing symptoms of depression.
Suppose you’re searching for an alternative to antidepressants, a non-drug solution for mental health support, or a cutting-edge approach to brain wellness in the Southeast valley. In that case, EXOMIND™ may be the breakthrough you’ve been looking for.
EXOMIND™ is a revolutionary transcranial magnetic stimulation (TMS) system engineered by BTL to offer mental health support in a completely non-invasive, drug-free format.
Unlike traditional antidepressant therapies, which may take weeks to show results and can come with side effects, EXOMIND™ directly stimulates areas of the brain involved in:
Using ExoTMS (Exogenous Transcranial Magnetic Stimulation), the device delivers focused electromagnetic pulses that enhance neuroplasticity—the brain’s ability to adapt, rebuild, and form new neural pathways.
Over time, these sessions help strengthen brain function, providing relief from depressive symptoms and promoting a sense of clarity and calm.
EXOMIND™ is FDA-cleared for adults with Major Depressive Disorder (MDD) who have not found success with conventional treatments like antidepressants or therapy alone. This gives patients access to an evidence-based, non-invasive mental health therapy with minimal side effects.
Benefits of FDA clearance:
A thorough consultation with one of our healthcare professionals will determine if EXOMIND™ is the right fit for your needs.